Haruhiko Morito
Tohoku University, Japan
Title: rystal growth of Na-Si clathrates by the flux method
Biography
Biography: Haruhiko Morito
Abstract
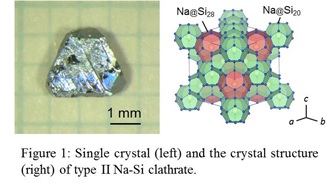
Introduction: Si clathrate compounds have been widely studied due to their unique open-framework structures of Si polyhedrons. Two types of Si clathrates encapsulating Na atoms have been known: type I (Na8Si46) and type II (NaxSi136, 0 < x ≤ 24). These Na-Si clathrates have been generally synthesized by thermal decomposition of a Na-Si binary compound, Na4Si4, at 673–823 K under high-vacuum conditions (<10−2 Pa), and the obtained samples were in the form of powder with a particle size in the micrometer range. The purpose of this study is the crystal growth of the type I and type II Na-Si clathrates by using a Na-Sn flux. Experimental: The starting material of a mixture of Na, Na4Si4, and Na15Sn4 was prepared by heating Na, Si, and Sn (molar ratio, Na/Si/Sn = 6:2:1) at 1173 K in Ar atmosphere. The mixture was heated at 673–873 K for 6–24 h in the container with a temperature gradient. After heating, air-sensitive compounds in the samples, such as Na-Sn compounds, were reacted with ethanol, and the water-soluble reactants were removed by washing with water. Sn present in the products or formed by the ethanol treatment was removed by dissolution in a dilute nitric acid aqueous solution. Results: The single crystals of type I clathrate were crystallized due to the evaporation of Na from the Na-Sn-Si solution at 673–773 K. Most of the single crystals had sizes of several hundred micrometers to 1 mm, and the maximum size reached to about 3 mm. Heating the starting mixture at 823–873 K resulted in the crystal growth of the type II clathrate. The single crystals having {111} habit planes grew up to about 2 mm in size as shown in Fig. 1.